Efficient and robust coupling methods for electro-mechanic models of the human heart
PIs: Prof. Christian Wieners, Prof. Axel Loewe
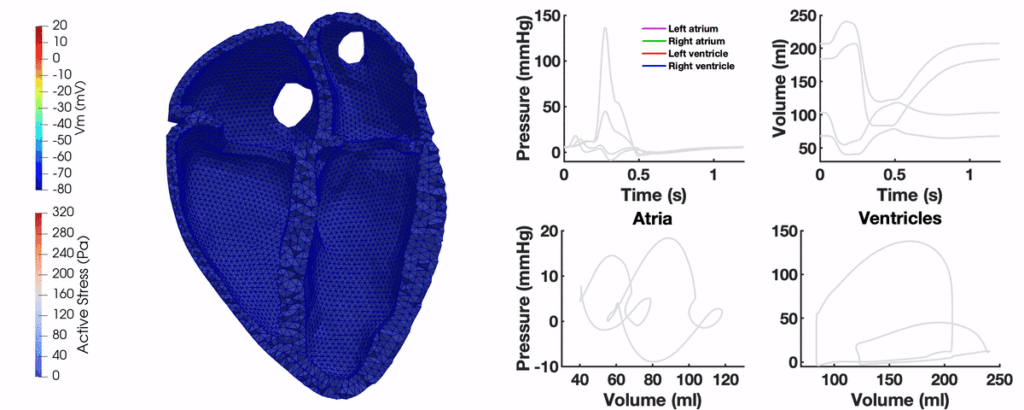
Aim:
This project aims to extend the limits of multi-physics simulations of the human heart by developing and evaluating robust coupling schemes for models of the cardiac electro-mechanical system with high biophysical accuracy across multiple scales and dimensions. By combining our profound interdisciplinary experience in modeling each of the cardiac physical systems separately with our preliminary work in coupling them, we are well positioned to construct and analyze an efficient and accurate parallel finiteelement realization of the fully coupled system.
As specific goals, we will develop and provide
(1) a formulation for a robustly coupled model for the whole heart comprising active electrophysiology, cardiac mechanics and circulatory function;
2) an efficient and scalable numerical implementation of this model and a comprehensive convergence analysis of the approximations in space and time;
(3) a user-friendly integration of the fully coupled model into the established openCARP simulation framework to foster community adoption of this research tool and pave the way for clinical translation.
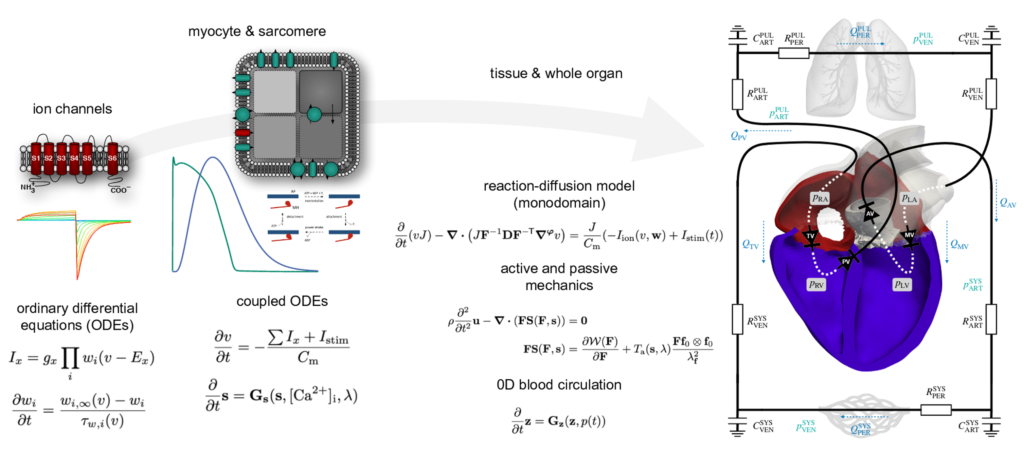
Model equations ordered in ascending spatial dimension. At the micro level, ion concentrations and resulting contraction forces are calculated using cell models. The propagation of the electrical stimulus is modeled using the monodomain equation and the deformation of the heart using the impulse balance and a so-called „active stress“ approach. The blood circulation is described using a closed 0D circulation model.
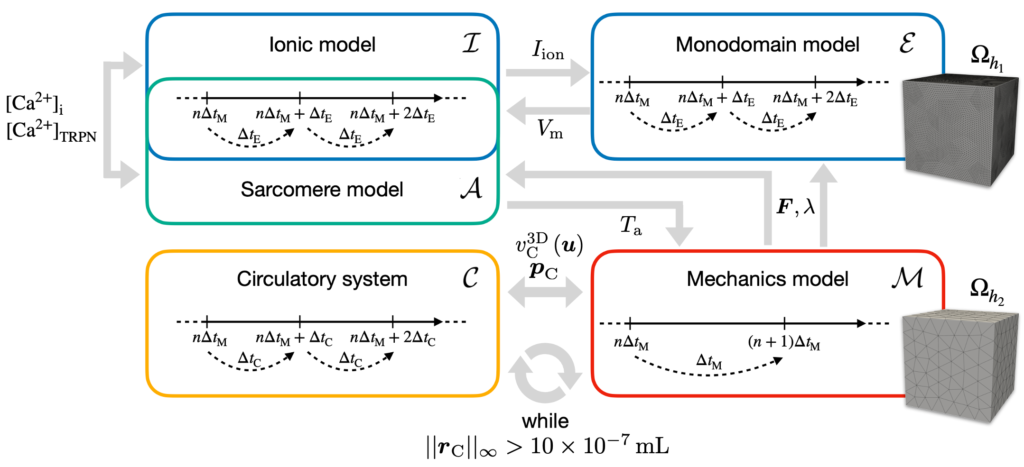
Components of the fully coupled model. The ion and sarcomere models describe the processes at cell level and are coupled via calcium concentrations. The monodomain model describes the electrical propagation of the stimulus across the organ using the finite element method and is linked to the ion models via the transmembrane voltage and the resulting ion currents across the cell membrane. The deformation is calculated using the solid mechanics finite element model based on the contraction force of the sarcomere models. Pressures and volumes within the atria and ventricles are determined using a circulatory model. The deformation of the mechanics model affects the electrophysiology via several mechano-electric feedback (MEF) mechanisms.Both the spatial and temporal resolution differs for the different model components, which is why stable and robust coupling methods are of great importance.
Description:
Background:
Cardiac diseases are the number one cause of death in Germany. Computational modeling of the cardiovascular system can help to understand the relevant mechanisms and help to tailor treatments for heart diseases. Although cardiac computational modeling has significantly advanced in the last decades, this is often limited to a single function such as electrophysiology, biomechanics, blood flow in the heart or the circulatory system. These single-physics approaches are very valuable research tools, but the interaction of cardiac functions is required to fully leverage the potential of in silico approaches.
Methods:
Our multi-physics model consists of several model components that describe the electromechanical contraction of the heart at different spatial scales. Ion concentrations within the cell are modeled via ion channels of the cardiomyocyte cell membrane. Based on the resulting calcium concentration, a contraction force is obtained using a sarcomere model. The activation of the myocardium is based on the electrical propagation across the organ, which – like the deformation of the heart – is calculated using the finite element method. A circulatory model is coupled to the deformation of the heart via the volumes and pressures of the atria and ventricles.
Building on our current work, we will further develop our multi-physics model by extending it with stable and efficient implicit-explicit time integration and finite element formulations and making the fully coupled system scalable. Subsequently, we will use this advanced model to study the effects of local electrical dyssynchrony and the application of cardiac resynchronization therapy in collaboration with our clinical partners. In addition, we will identify application scenarios where the highly complex full model can be reduced and quantify the resulting uncertainty.
Involved Institutions:
Karlsruhe Institute of Technology
Institute of Biomedical Engineering
Computational Cardiac Modeling
Karlsruhe Institute of Technology
Institute for Applied and Numerical Mathematics
Scientific Computing
Applicants:

Prof. Dr. Christian Wieners

PD Dr.-Ing. Axel Loewe
M. Sc. Laura Stengel

M. Sc. Jonathan Krauß
Publications
2024
Stengel, Laura; Krauß, Jonathan; Loewe, Axel; Wieners, Christian
Enriched and Discontinuous Galerkin Discretizations for a Cardiac Mechanics Benchmark Problem Artikel
In: 2024.
@article{Stengel2024,
title = {Enriched and Discontinuous Galerkin Discretizations for a Cardiac Mechanics Benchmark Problem},
author = {Laura Stengel and Jonathan Krauß and Axel Loewe and Christian Wieners},
url = {https://cinc.org/archives/2024/pdf/CinC2024-299.pdf},
doi = {doi.org/10.22489/CinC.2024.299},
year = {2024},
date = {2024-12-31},
urldate = {2024-12-31},
abstract = {Computer models of the human heart enhance understanding of cardiac function but require a compromise between computational cost and numerical accuracy for clinical use. Due to the high mathematical complexity of the underlying model, the commonly used finite element discretization may not achieve the optimal balance between efficiency, reliability, and accuracy. To investigate the impact of different spatial discretization schemes on cardiac mechanics, we realized a benchmark configuration, which considers the hyper-elastic problem of inflating and ac-
tively contracting an idealized left ventricle with transversely isotropic and nearly incompressible properties. Comparing linear and quadratic conforming Galerkin, discontinuous Galerkin and enriched Galerkin elements, we found that enriched and discontinuous Galerkin methods reduced locking phenomena but with higher numbers of degrees of freedom and computational costs, particularly for the discontinuous Galerkin approach. However, enriched Galerkin demonstrated comparable robustness to discontinuous Galerkin with substantially fewer degrees of freedom, presenting a favorable compromise between computational efficiency and numerical robustness.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
tively contracting an idealized left ventricle with transversely isotropic and nearly incompressible properties. Comparing linear and quadratic conforming Galerkin, discontinuous Galerkin and enriched Galerkin elements, we found that enriched and discontinuous Galerkin methods reduced locking phenomena but with higher numbers of degrees of freedom and computational costs, particularly for the discontinuous Galerkin approach. However, enriched Galerkin demonstrated comparable robustness to discontinuous Galerkin with substantially fewer degrees of freedom, presenting a favorable compromise between computational efficiency and numerical robustness.
Duhme, Jan-Erik; Krauß, Jonathan; Loewe, Axel
Modeling Human Ventricular Cardiomyocyte Force-Frequency Relationship Artikel
In: Current Directions in Biomedical Engineering, Bd. 10, Ausg. 4, S. 212-215, 2024.
@article{Duhme2024,
title = {Modeling Human Ventricular Cardiomyocyte Force-Frequency Relationship},
author = {Jan-Erik Duhme and Jonathan Krauß and Axel Loewe},
editor = {De Gruyter Bril},
url = {https://www.degruyterbrill.com/document/doi/10.1515/cdbme-2024-2051/html},
doi = {doi.org/10.1515/cdbme-2024-2051},
year = {2024},
date = {2024-12-19},
urldate = {2024-12-19},
journal = {Current Directions in Biomedical Engineering},
volume = {10},
issue = {4},
pages = {212-215},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Stengel, Laura; Krauß, Jonathan; Loewe, Axel; Wieners, Christian
Enriched and Discontinuous Galerkin Discretizations for a Cardiac Mechanics Benchmark Problem Artikel
In: Computing in Cardiology , Bd. 51, 2024, ISBN: 2325-887X .
@article{Stengel2024b,
title = {Enriched and Discontinuous Galerkin Discretizations for a Cardiac Mechanics Benchmark Problem},
author = {Laura Stengel and Jonathan Krauß and Axel Loewe and Christian Wieners},
url = {https://www.cinc.org/archives/2024/pdf/CinC2024-299.pdf},
doi = {doi.org/10.22489/CinC.2024.299},
isbn = {2325-887X },
year = {2024},
date = {2024-10-28},
urldate = {2024-10-28},
journal = {Computing in Cardiology },
volume = {51},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Gerach, Tobias; Loewe, Axel
In: The Journal of Physiology, 2024.
@article{Gerach2024,
title = {Differential effects of mechano-electric feedback mechanisms on whole-heart activation, repolarization, and tension},
author = {Tobias Gerach and Axel Loewe},
editor = {John Wiley The Physiological Society},
url = {https://physoc.onlinelibrary.wiley.com/doi/10.1113/JP285022},
doi = {https://doi.org/10.1113/JP285022},
year = {2024},
date = {2024-01-07},
urldate = {2024-01-07},
journal = {The Journal of Physiology},
abstract = {The human heart is subject to highly variable amounts of strain during day-to-day activities and needs to adapt to a wide range of physiological demands. This adaptation is driven by an autoregulatory loop that includes both electrical and the mechanical components. In particular, mechanical forces are known to feed back into the cardiac electrophysiology system, which can result in pro- and anti-arrhythmic effects. Despite the widespread use of computational modelling and simulation for cardiac electrophysiology research, the majority of in silico experiments ignore this mechano-electric feedback entirely due to the high computational cost associated with solving cardiac mechanics. In this study, we therefore use an electromechanically coupled whole-heart model to investigate the differential and combined effects of electromechanical feedback mechanisms with a focus on their physiological relevance during sinus rhythm. In particular, we consider troponin-bound calcium, the effect of deformation on the tissue diffusion tensor, and stretch-activated channels. We found that activation of the myocardium was only significantly affected when including deformation into the diffusion term of the monodomain equation. Repolarization, on the other hand, was influenced by both troponin-bound calcium and stretch-activated channels and resulted in steeper repolarization gradients in the atria. The latter also caused afterdepolarizations in the atria. Due to its central role for tension development, calcium bound to troponin affected stroke volume and pressure. In conclusion, we found that mechano-electric feedback changes activation and repolarization patterns throughout the heart during sinus rhythm and lead to a markedly more heterogeneous electrophysiological substrate.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Fröhlich, Jonathan; Gerach, Tobias; Krauß, Jonathan; Loewe, Axel; Stengel, Laura; Wieners, Christian
In: GAMM Mitteilungen, Bd. 46, Ausg. 2, 2024, ISSN: 1522-2608.
@article{Fröhlich2024,
title = {Numerical evaluation of elasto-mechanical and visco-elastic electro-mechanical models of the human heart},
author = {Jonathan Fröhlich and Tobias Gerach and Jonathan Krauß and Axel Loewe and Laura Stengel and Christian Wieners},
editor = {Gesellschaft Mechanik},
url = {https://onlinelibrary.wiley.com/doi/10.1002/gamm.202370010},
doi = {https://doi.org/10.1002/gamm.202370010},
issn = {1522-2608},
year = {2024},
date = {2024-01-02},
urldate = {2024-01-02},
journal = {GAMM Mitteilungen},
volume = {46},
issue = {2},
abstract = {We investigate the properties of static mechanical and dynamic electro-mechanical models for the deformation of the human heart. Numerically this is realized by a staggered scheme for the coupled partial/ordinary differential equation (PDE-ODE) system. First, we consider a static and purely mechanical benchmark configuration on a realistic geometry of the human ventricles. Using a penalty term for quasi-incompressibility, we test different parameters and mesh sizes and observe that this approach is not sufficient for lowest order conforming finite elements. Then, we compare the approaches of active stress and active strain for cardiac muscle contraction. Finally, we compare in a coupled anatomically realistic electro-mechanical model numerical Newmark damping with a visco-elastic model using Rayleigh damping. Nonphysiological oscillations can be better mitigated using viscosity.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2023
Krauß, Jonathan; Gerach, Tobias; Loewe, Axel
Effects of Ventricular Myofiber Orientation on Mechanical Function in Human Heart Simulations Artikel
In: Computing in Cardiology , Bd. 49, 2023, ISBN: 325-887X.
@article{Krauß2023,
title = {Effects of Ventricular Myofiber Orientation on Mechanical Function in Human Heart Simulations},
author = {Jonathan Krauß and Tobias Gerach and Axel Loewe},
url = {https://www.cinc.org/archives/2022/pdf/CinC2022-132.pdf},
doi = {0.22489/CinC.2022.132},
isbn = {325-887X},
year = {2023},
date = {2023-11-27},
urldate = {2023-11-27},
journal = {Computing in Cardiology },
volume = {49},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Gerach, Tobias; Krauß, Jonathan; Schuler, Steffen; Loewe, Axel
In: Computing in Cardiology , Bd. 50, 2023, ISBN: 325-887X .
@article{Gerach2023,
title = {Whole Heart Simulation of Severe Aortic Stenosis Using a Lumped Parameter Model of Heart Valve Dynamics},
author = {Tobias Gerach and Jonathan Krauß and Steffen Schuler and Axel Loewe},
url = {https://www.cinc.org/archives/2023/pdf/CinC2023-162.pdf},
doi = {doi.org/10.22489/CinC.2023.162},
isbn = {325-887X },
year = {2023},
date = {2023-10-30},
urldate = {2023-10-30},
journal = {Computing in Cardiology },
volume = {50},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Krauß, Jonathan; Gerach, Tobias; Loewe, Axel
Comparison of Pericardium Modeling Approaches for Mechanical Whole Heart Simulations Artikel
In: Computing in Cardiology , Bd. 50, 2023, ISSN: 2325-887X.
@article{Krauß2023b,
title = {Comparison of Pericardium Modeling Approaches for Mechanical Whole Heart Simulations},
author = {Jonathan Krauß and Tobias Gerach and Axel Loewe},
url = {https://www.cinc.org/archives/2023/pdf/CinC2023-150.pdf},
doi = {10.22489/CinC.2023.150},
issn = {2325-887X},
year = {2023},
date = {2023-10-23},
journal = {Computing in Cardiology },
volume = {50},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
