Heart hemodynamics modelling using Fluid-Structure-Interaction and statistical shape model
PIs: Prof. Leonid Goubergrits, Prof. Titus Kühne


Aim:
The aim of the project is to enable patient-specific simulations of cardiac hemodynamics to assist physicians in diagnosis and therapy. The proposed approach combines numerical flow simulations using fluid-structure coupling as well as statistical shape models based on MRI or CT image data.
Description:
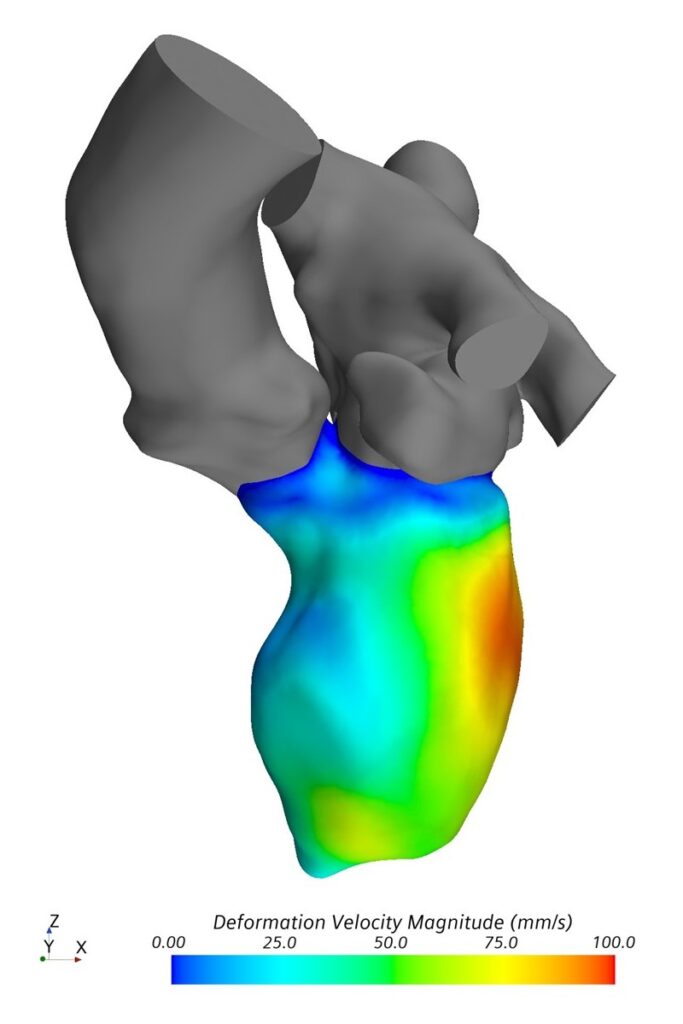
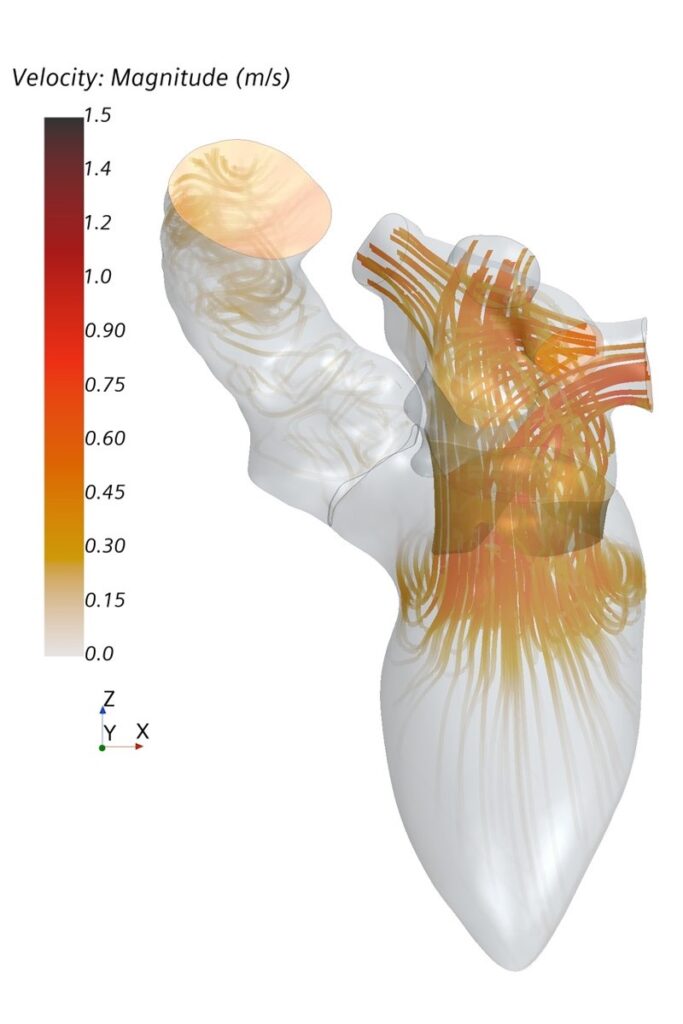
Analysis of cardiac hemodynamics plays an increasing role in diagnosis and treatment of heart diseases. A routine assessment of complex parameters proposed as hemodynamic biomarkers for cardiovascular diseases is currently difficult. However, numerical modeling allows calculation of spatially and temporally resolved information of patient-specific heart function.
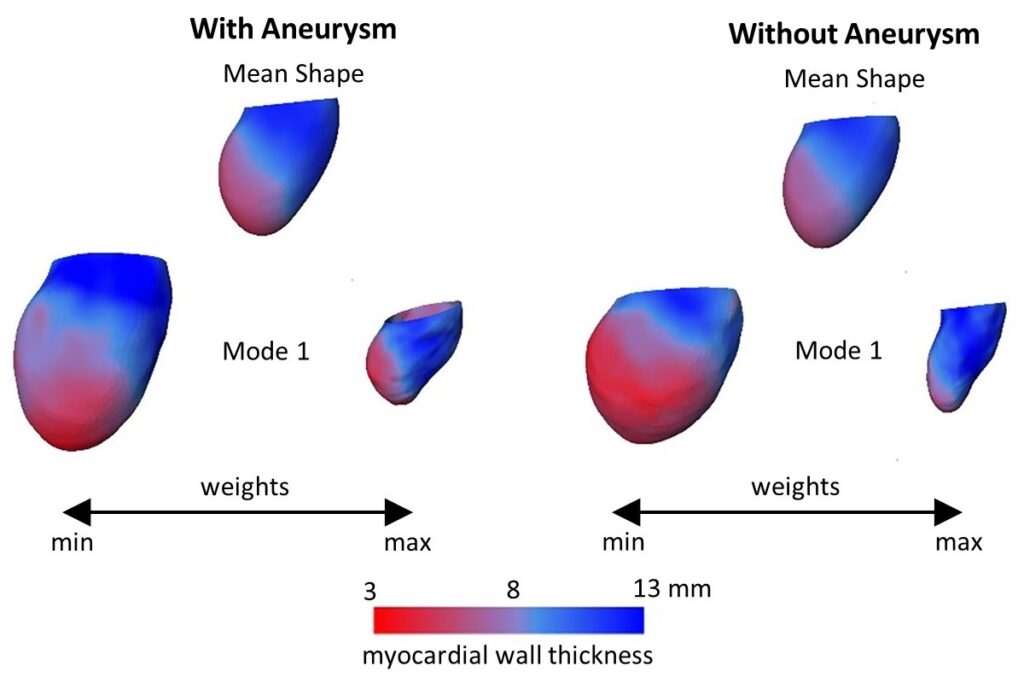
The major aim of this project is to allow patient-specific simulations under clinical conditions and thus support physicians during clinical routine. The proposed numerical approach will combine computational fluid dynamics (CFD) and statistical shape models (SSM). SSM allow the description of patient-specific geometries as a superposition of weighted shape modes. It is therefore possible to create a standardized simulation of the left ventricle based on an average shape. Different imaging modalities, namely CT, MRI and echocardiography, are used to parameterize the simulation. The ventricular contraction is modelled using a prescribed motion, meaning the contraction is acquired from image data. The proposed CFD-SSM numerical approach is flexible and allows identification of an optimal model complexity: simple enough to be applicable in a clinical setting and complex enough to provide required level of information for precise diagnosis and treatment decision.
The approach should enable robust and fast simulation of cardiac hemodynamics for clinicians. It has the potential to allow complete automation of patient-specific modelling. Automation will improve usability and minimize all user-related errors. The initial clinical focus of the project are heart valve diseases. The successful realization of the project requires close cooperation between clinical and engineering scientists, which is well established at the Institute of Cardiovascular Computer-assisted Medicine at Charité – Universitätsmedizin Berlin.
Involved Institutions:
Charité – Universitätsmedizin Berlin
Institute of Computer-assisted Cardiovascular Medicine
Links:
Applicants:

Prof. Dr.-Ing. Leonid Goubergrits

Prof. Dr. med. Titus Kühne
Publications
2024
Obermeier, Lukas; Wiegand, M.; Hellmeier, F.; Manini, C.; Kuehne, Titus; Goubergrits, Leonid
Verification Study of In Silico Computed Intracardiac Blood Flow With 4D Flow MRI Artikel
In: IEEE Transactions on Biomedical Engineering, Bd. 71, Ausg. 9, S. 2568 - 2579, 2024.
@article{nokeyc,
title = {Verification Study of In Silico Computed Intracardiac Blood Flow With 4D Flow MRI},
author = {Lukas Obermeier and M. Wiegand and F. Hellmeier and C. Manini and Titus Kuehne and Leonid Goubergrits},
editor = {IEEE},
url = {https://ieeexplore.ieee.org/document/10478556},
doi = {10.1109/TBME.2024.3381212},
year = {2024},
date = {2024-03-25},
urldate = {2024-03-25},
journal = {IEEE Transactions on Biomedical Engineering},
volume = {71},
issue = {9},
pages = {2568 - 2579},
abstract = {Objective: Major challenges for clinical applications of in silico medicine are limitations in time and computational resources. Computational approaches should therefore be tailored to specific applications with relatively low complexity and must be verified and validated against clinical gold standards. Methods: This study performed computational fluid dynamics simulations of left ventricular hemodynamics of different complexity based on shape reconstruction from steady state gradient echo magnetic resonance imaging (MRI) data. Computed flow results of a rigid wall model (RWM) and a prescribed motion fluid-structure interaction (PM-FSI) model were compared against phase-contrast MRI measurements for three healthy subjects. Results: Extracted boundary conditions from the steady state MRI sequences as well as computed metrics, such as flow rate, valve velocities, and kinetic energy show good agreement with in vivo flow measurements. Regional flow analysis reveals larger differences. Conclusion: Basic flow structures are well captured with RWM and PM-FSI. For the computation of further biomarkers like washout or flow efficiency, usage of PM-FSI is required. Regarding boundary-near flow, more accurate anatomical models are inevitable. Significance: These results delineate areas of application of both methods and lay a foundation for larger validation studies and sensitivity analysis for healthy and diseased cases, being an essential step upon clinical translations.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Obermeier, Lukas; Korte, Jana; Vellguth, Katharina; Barbieri, Fabian; Hellmeier, Florian; Berg, Philipp; Goubergrits, Leonid
In: GAMM-Mitteilungen, 2024.
@article{nokeyg,
title = {Inter-model and inter-modality analysis of left ventricular hemodynamics: comparative study of two CFD approaches based on TTE and MRI},
author = {Lukas Obermeier and Jana Korte and Katharina Vellguth and Fabian Barbieri and Florian Hellmeier and Philipp Berg and Leonid Goubergrits},
url = {https://onlinelibrary.wiley.com/doi/full/10.1002/gamm.202370004},
doi = {10.1002/gamm.202370004},
year = {2024},
date = {2024-01-23},
urldate = {2024-01-23},
journal = {GAMM-Mitteilungen},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2022
Hellmeier, Florian; Bruening, Jan; Berg, Philipp; Saalfeld, Sylvia; Spuler, Andreas; Sandalcioglu, Erol I.; Beuing, Oliver; Larsen, Naomi; Schaller, Jens; Goubergrits, Leonid
In: BMJ Open, Bd. 12, Ausg. 11, 2022.
@article{10.1136/bmjopen-2022-063051,
title = {Geometric uncertainty in intracranial aneurysm rupture status discrimination: a two-site retrospective study},
author = {Florian Hellmeier and Jan Bruening and Philipp Berg and Sylvia Saalfeld and Andreas Spuler and Erol I. Sandalcioglu and Oliver Beuing and Naomi Larsen and Jens Schaller and Leonid Goubergrits},
editor = {BMJ Open},
url = {https://pubmed.ncbi.nlm.nih.gov/36351732/},
doi = {10.1136/bmjopen-2022-063051},
year = {2022},
date = {2022-11-08},
urldate = {2022-11-08},
journal = {BMJ Open},
volume = {12},
issue = {11},
abstract = {Assessing the risk associated with unruptured intracranial aneurysms (IAs) is essential in clinical decision making. Several geometric risk parameters have been proposed for this purpose. However, performance of these parameters has been inconsistent. This study evaluates the performance and robustness of geometric risk parameters on two datasets and compare it to the uncertainty inherent in assessing these parameters and quantifies interparameter correlations.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Schlief, Adriano; Bruening, Jan; Voß, Samuel; Berg, Philipp; Goubergrits, Leonid
Uncertainty Quantification of Hemodynamic Parameters for Cerebral Aneurysm Rupture Risk Assessment Proceedings Article
In: Virtual Physiological Human Conference (VPH) Porto, Portugal, 2022.
@inproceedings{nokeyu,
title = {Uncertainty Quantification of Hemodynamic Parameters for Cerebral Aneurysm Rupture Risk Assessment},
author = {Adriano Schlief and Jan Bruening and Samuel Voß and Philipp Berg and Leonid Goubergrits},
url = {https://www.lss.ovgu.de/lss/en/Publications.html},
year = {2022},
date = {2022-09-12},
urldate = {2022-09-12},
address = {Porto, Portugal},
organization = {Virtual Physiological Human Conference (VPH)},
keywords = {},
pubstate = {published},
tppubtype = {inproceedings}
}
Goubergrits, Leonid; Vellguth, Katharina; Obermeier, Lukas; Schlief, Adriano; Tautz, Lennart; Bruening, Jan; Lamecker, Hans; Szengel, Angelika; Nemchyna, Olena; Knosalla, Christoph; Kuehne, Titus; Solowjowa, Natalia
In: Front. Cardiovasc. Med., 05 July 2022, Bd. Sec. Cardiovascular Imaging, Ausg. Volume 9 - 2022, S. 901902, 2022.
@article{10.3389/fcvm.2022.901902,
title = {CT-Based Analysis of Left Ventricular Hemodynamics Using Statistical Shape Modeling and Computational Fluid Dynamics},
author = {Leonid Goubergrits and Katharina Vellguth and Lukas Obermeier and Adriano Schlief and Lennart Tautz and Jan Bruening and Hans Lamecker and Angelika Szengel and Olena Nemchyna and Christoph Knosalla and Titus Kuehne and Natalia Solowjowa},
editor = {Frontiers Cardiovascular Medicine},
url = {https://www.frontiersin.org/articles/10.3389/fcvm.2022.901902/full},
doi = {10.3389/fcvm.2022.901902“},
year = {2022},
date = {2022-07-05},
urldate = {2022-07-05},
journal = {Front. Cardiovasc. Med., 05 July 2022},
volume = {Sec. Cardiovascular Imaging},
issue = {Volume 9 - 2022},
pages = {901902},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Obermeier, Lukas; Vellguth, Katharina; Schlief, Adriano; Tautz, Lennart; Bruening, Jan; Knosalla, Christoph; Kuehne, Titus; Solowjowa, Natalia; Goubergrits, Leonid
In: Frontiers in Cardiovascular Medicine, Ausg. 9/2022, 2022, ISSN: 2297-055X.
@article{Obermeier2022,
title = {CT-Based Simulation of Left Ventricular Hemodynamics: A Pilot Study in Mitral Regurgitation and Left Ventricle Aneurysm Patients},
author = {Lukas Obermeier and Katharina Vellguth and Adriano Schlief and Lennart Tautz and Jan Bruening and Christoph Knosalla and Titus Kuehne and Natalia Solowjowa and Leonid Goubergrits},
doi = {10.3389/fcvm.2022.828556},
issn = {2297-055X},
year = {2022},
date = {2022-03-22},
urldate = {2022-03-22},
journal = {Frontiers in Cardiovascular Medicine},
issue = {9/2022},
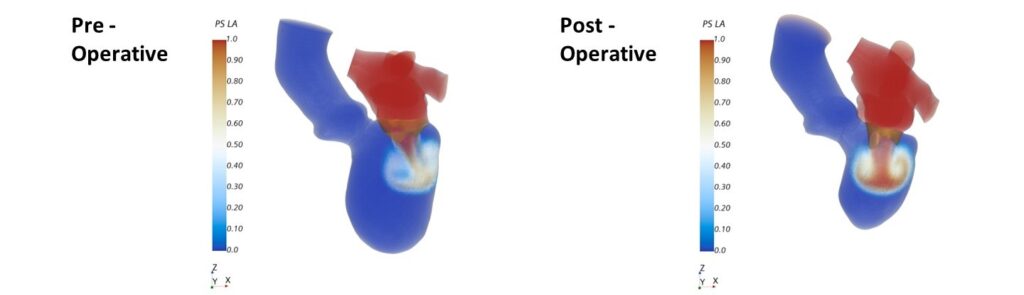
abstract = {Background: Cardiac CT (CCT) is well suited for a detailed analysis of heart structures due to its high spatial resolution, but in contrast to MRI and echocardiography, CCT does not allow an assessment of intracardiac flow. Computational fluid dynamics (CFD) can complement this shortcoming. It enables the computation of hemodynamics at a high spatio-temporal resolution based on medical images. The aim of this proposed study is to establish a CCT-based CFD methodology for the analysis of left ventricle (LV) hemodynamics and to assess the usability of the computational framework for clinical practice.
Materials and methods: The methodology is demonstrated by means of four cases selected from a cohort of 125 multiphase CCT examinations of heart failure patients. These cases represent subcohorts of patients with and without LV aneurysm and with severe and no mitral regurgitation (MR). All selected LVs are dilated and characterized by a reduced ejection fraction (EF). End-diastolic and end-systolic image data was used to reconstruct LV geometries with 2D valves as well as the ventricular movement. The intraventricular hemodynamics were computed with a prescribed-motion CFD approach and evaluated in terms of large-scale flow patterns, energetic behavior, and intraventricular washout.
Results: In the MR patients, a disrupted E-wave jet, a fragmentary diastolic vortex formation and an increased specific energy dissipation in systole are observed. In all cases, regions with an impaired washout are visible. The results furthermore indicate that considering several cycles might provide a more detailed view of the washout process. The pre-processing times and computational expenses are in reach of clinical feasibility.
Conclusion: The proposed CCT-based CFD method allows to compute patient-specific intraventricular hemodynamics and thus complements the informative value of CCT. The method can be applied to any CCT data of common quality and represents a fair balance between model accuracy and overall expenses. With further model enhancements, the computational framework has the potential to be embedded in clinical routine workflows, to support clinical decision making and treatment planning.
Keywords: cardiac computed tomography; computational fluid dynamics; fluid-structure interaction; image-based modeling; intraventricular hemodynamics; left ventricle aneurysm; mitral regurgitation.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Materials and methods: The methodology is demonstrated by means of four cases selected from a cohort of 125 multiphase CCT examinations of heart failure patients. These cases represent subcohorts of patients with and without LV aneurysm and with severe and no mitral regurgitation (MR). All selected LVs are dilated and characterized by a reduced ejection fraction (EF). End-diastolic and end-systolic image data was used to reconstruct LV geometries with 2D valves as well as the ventricular movement. The intraventricular hemodynamics were computed with a prescribed-motion CFD approach and evaluated in terms of large-scale flow patterns, energetic behavior, and intraventricular washout.
Results: In the MR patients, a disrupted E-wave jet, a fragmentary diastolic vortex formation and an increased specific energy dissipation in systole are observed. In all cases, regions with an impaired washout are visible. The results furthermore indicate that considering several cycles might provide a more detailed view of the washout process. The pre-processing times and computational expenses are in reach of clinical feasibility.
Conclusion: The proposed CCT-based CFD method allows to compute patient-specific intraventricular hemodynamics and thus complements the informative value of CCT. The method can be applied to any CCT data of common quality and represents a fair balance between model accuracy and overall expenses. With further model enhancements, the computational framework has the potential to be embedded in clinical routine workflows, to support clinical decision making and treatment planning.
Keywords: cardiac computed tomography; computational fluid dynamics; fluid-structure interaction; image-based modeling; intraventricular hemodynamics; left ventricle aneurysm; mitral regurgitation.
