Multi-scale algorithms and simulation for the patient-specific optimization of endovascular interventions in cerebral aneurysms
PIs: Prof. Alexander Popp, Prof. Barbara Wohlmuth, Prof. Jan Kirschke

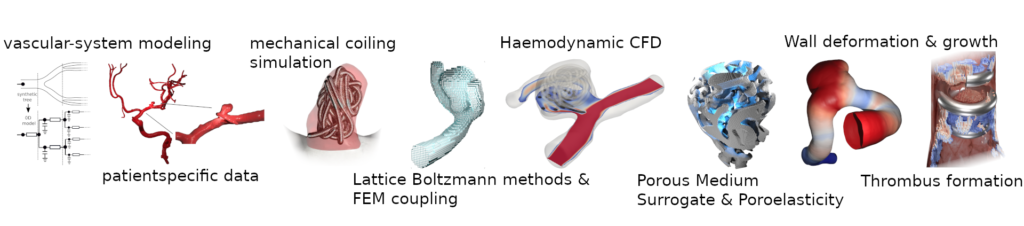
Aim:
Scientific objective
The main objective of this project is to develop methods and simulation tools that map the course of endovascular cerebral aneurysm treatment and support interventionalists in treatment planning – from diagnosis to intervention optimization and assessment of the long-term perspective.
Outreach & Networking: In addition to the purely scientific aspect, cooperation within, but also networking beyond the boundaries of the SPP is a central goal, which we pursue in the hope of bringing together scientists and physicians with clinical/practical experience in order to strengthen the active exchange between these groups on the topic of cerebral aneurysm treatment in Germany. For our organized thematic workshops, see below under “Activities within the SPP”, as well as under “Collaborations” for our networking and collaboration activities within the SPP and internationally.
Teaching & training: With the vision of a practically applicable tool for doctors in everyday clinical practice, our coil simulation tool is already being used by Prof. Leonid Goubergrits (Charité Berlin) in the training of students, who can use it to perform and assess virtual coiling.
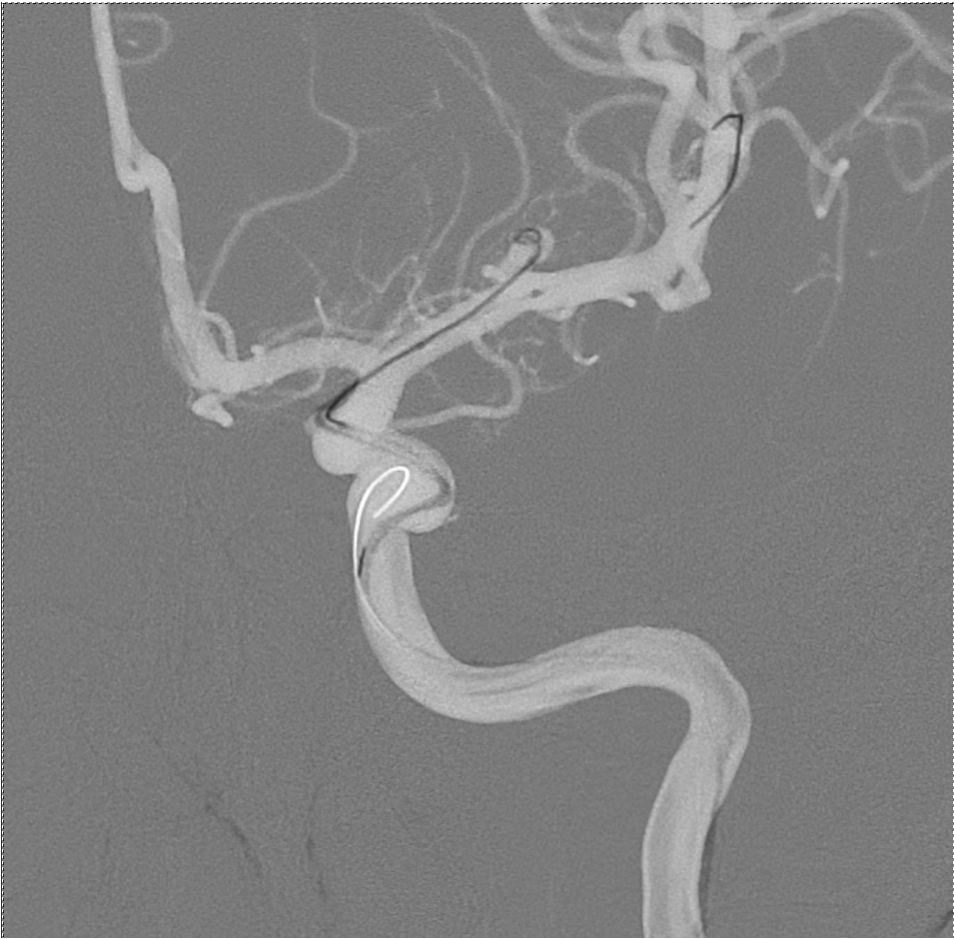
Angiography image during an aneurysm coiling procedure.
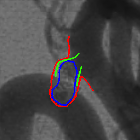
Relapse of a coiled aneurysm.
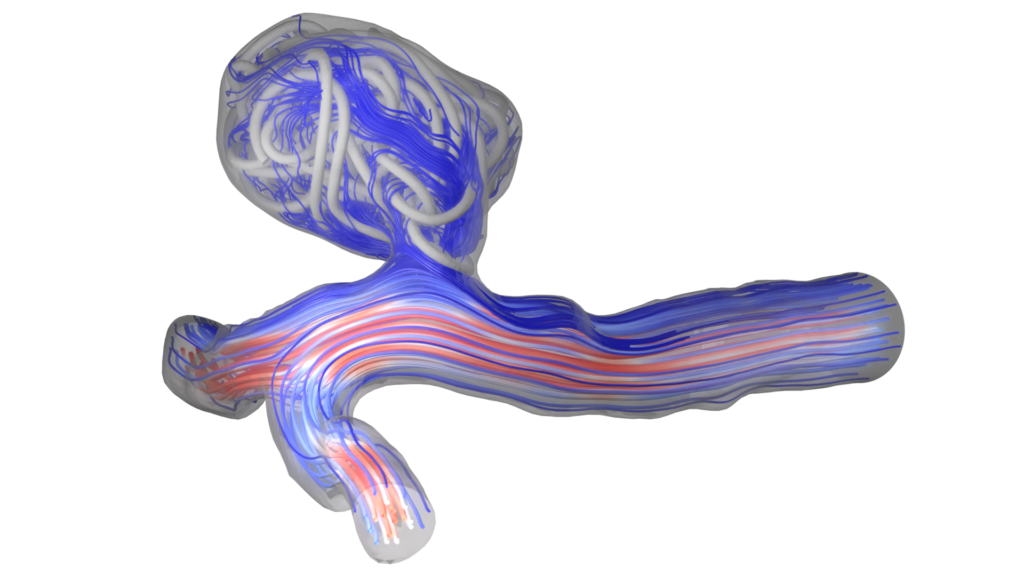
Streamlines through a coil configuration
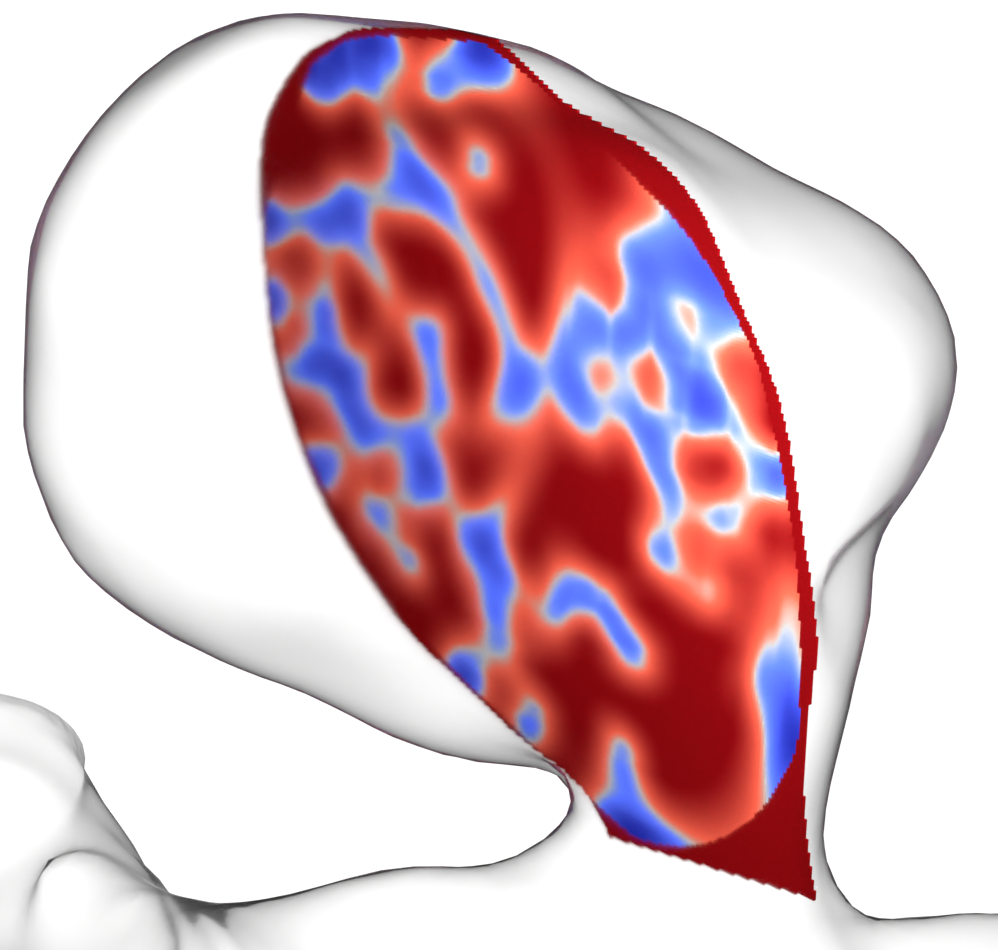
Cross-section through the porosity field of a coil
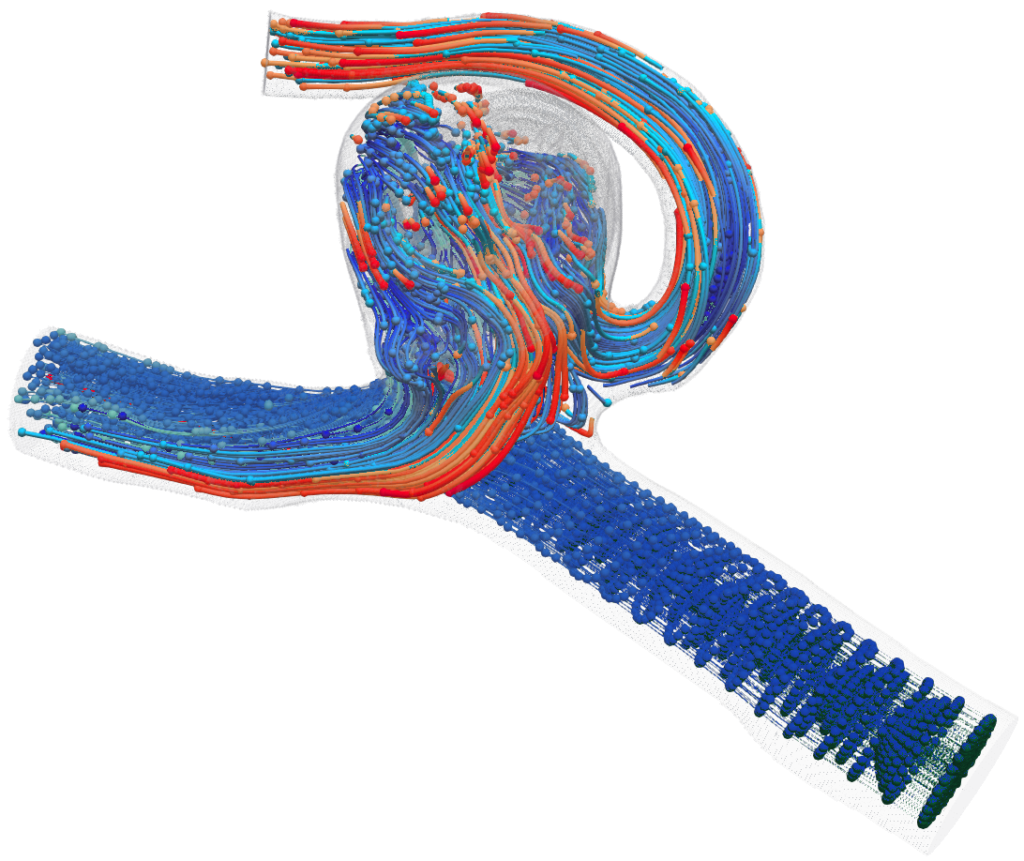
Tracer particles in flow through a coiled aneurysm
Description:
Medical background:
Definition and relevance: Cerebral aneurysms are protrusions of blood vessels in the brain, often caused by high blood pressure or arteriosclerosis, whose wall structure is exposed to an increased risk of rupture. If such an aneurysm ruptures, this can have drastic consequences for the patient, with almost half a million fatal cases every year. With a prevalence of around 3% in the EU, there is a high urgency for the further development of diagnostic and treatment methods.
Treatment: If an aneurysm is detected during an angiographic, CT or MRI examination, a decision must be made as to whether and, if so, how it should be treated. In particular, endovascular techniques such as aneurysm coiling or the insertion of flow diverters are increasingly being considered.
– Coiling: Coiling involves inserting an extremely soft microwire into the aneurysm via a catheter until it is filled as volumetrically as possible. The coil thus formed in the aneurysm prevents or at least reduces further blood flow in the critical area, with the formation of a thrombus around the coil further enhancing this effect.
– Flow-Diverter: A flow diverter is a very narrow-meshed stent and is placed in the vessel in front of the aneurysm. It directs the blood flow past the aneurysm, also with the aim of reducing the blood flow in the aneurysm, whereby the position in the vessel is held by pressing firmly against the vessel wall.
Risks and long-term prognosis: When selecting the specific endovascular device, e.g. when choosing a suitable coil, many parameters play a role, from length, thickness and insertion position to complex microstructures in the coil material. Risks are, for example, that parts of the coil protrude from the aneurysm if it is not placed ideally or that parts of the aneurysm are not completely covered or closed. Such cases are categorized according to the Raymond-Roy classification. The consequences of such incomplete occlusion can be residual blood flow in the aneurysm with pulsation-induced further weakening of the wall and even regrowth of the aneurysm, but also the formation of edema in the tissue behind the aneurysm. However, even in the case of a completely occluded aneurysm, the inflammation of the aneurysm wall or the complex interaction of the device material with the thrombus that forms can lead to shrinkage of the coil (coil compactification) with similar long-term consequences.
This makes the analysis of the long-term prognosis of treated aneurysms an essential aspect of research, in addition to the optimization of direct intervention.
Methods:
Using appropriate multi-scale models, this project selects optimal treatment strategies for endovascular intervention, including in-silico testing of different devices or surgical approaches, and predicts long-term treatment outcomes. For this purpose, porous media approaches are used as surrogate models for endovascular devices and for bio-active thrombus modeling, mixed-dimensional models for fluid-structure (vessel wall pulsation), structure-structure (device-wall interaction) and structure-solid (vessel wall-tissue) coupling. Highly parallel Lattice-Boltzmann solvers coupled with finite elements ensure the efficient numerical solution of the resulting equations even in realistic scenarios.
Phase I: In the first phase of the SPP project, the focus is on direct intervention planning. This includes the realistic simulation of blood flow dynamics in patient-specific geometries with aneurysm position-dependent boundary conditions and evaluation of relevant parameters such as wall shear stress for rupture risk analysis. The modeling of endovascular devices, including the statistical consideration of uncertainties, is also a decisive aspect in order to be able to make clinically relevant statements on the optimal choice of devices. Porous media and poroelastic approaches can be used to model and evaluate the influence of the devices used on the bluff flow dynamics and thus their occlusion quality. They also provide a basis for modeling and simulating thrombus formation, which will be another core component of the second project phase. The Lattice-Boltzmann to finite element coupling in the direction of vessel wall pulsation and dynamics and their implementation in corresponding high-performance frameworks will also lay the foundation for correspondingly more complex models in phase II.
Phase II: In Phase II, the focus is on the long-term perspective of the patient and the prediction of recurrence. The in-silico Raymond-Roy occlusion quality criteria developed in Phase I will be calibrated and validated using experimental data from our cooperation partners. In the field of endovascular devices, our complexity-reduced mechanical models are extended to include bioactive components such as HydroCoils or coated flow diverters, and structural-structural interactions with the vessel wall are included in addition to the fluid. Based on a reduced wall model, biomechanical models are implemented with inflammation processes that change the material properties of the wall. Our implementation of coupled fluid-structure interaction using Lattice-Boltzmann/Finite elements is extended to a fluid-structure-solid-state approach that considers the outer tissue to capture the edema formation process and the thrombus inside. To quantify and predict potential coil compaction, a phenomenological contraction model for the thrombus is developed and coupled with wall and coil mechanics. To improve clinical applicability in terms of simulation speed and to provide practical benefits to neurointerventionalists, machine learning will be used as a predictive tool.
Involved Institutions:
Links:
Applicants:

Prof. Dr. rer. nat. Barbara Wohlmuth

Prof. Dr.-Ing. Alexander Popp

Prof. Dr. med. Jan Kirschke

Fabian Holzberger

Medeea Horvat

Martin Frank, M.Sc.

Dr. rer. nat. Markus Muhr

Dr.-Ing. Matthias Mayr

Dr. med. Julian Schwarting
Publications
2024
Schwarting, Julian; Holzberger, Fabian; Muhr, Markus; Renz, Martin; Boeckh-Behrens, Tobias; Wohlmuth, Barbara; Kirschke, Jan
In: [PrePrint], arXiv, 2024.
@article{schwarting24aneurysm,
title = {Numerical simulation of individual coil placement – A proof-of-concept study for the prediction of recurrence after aneurysm coiling},
author = {Julian Schwarting and Fabian Holzberger and Markus Muhr and Martin Renz and Tobias Boeckh-Behrens and Barbara Wohlmuth and Jan Kirschke},
editor = {arXiv},
url = {https://arxiv.org/abs/2403.06889},
doi = {https://doi.org/10.48550/arXiv.2403.06889},
year = {2024},
date = {2024-03-11},
journal = {[PrePrint], arXiv},
abstract = {Rupture of intracranial aneurysms results in severe subarachnoidal hemorrhage, which is associated with high morbidity and mortality. Neurointerventional occlusion of the aneurysm through coiling has evolved to a therapeutical standard. The choice of the specific coil has an important influence on secondary regrowth requiring retreatment. Aneurysm occlusion was simulated either through virtual implantation of a preshaped 3D coil or with a porous media approach. In this study, we used a recently developed numerical approach to simulate aneurysm shapes in specific challenging aneurysm anatomies and correlated these with aneurysm recurrence 6 months after treatment. The simulation showed a great variety of coil shapes depending on the variability in possible microcatheter positions. Aneurysms with a later recurrence showed a tendency for more successful coiling attempts. Results revealed further trends suggesting lower simulated packing densities in aneurysms with reoccurrence. Simulated packing densities did not correlate with those calculated by conventional software, indicating the potential for our approach to offer additional predictive value. Our study, therefore, pioneers a comprehensive numerical model for simulating aneurysm coiling, providing insights into individualized treatment strategies and outcome prediction. Future directions involve expanding the model's capabilities to simulate intraprocedural outcomes and long-term predictions, aiming to refine occlusion quality criteria and validate prediction parameters in larger patient cohorts. This simulation framework holds promise for enhancing clinical decision-making and optimizing patient outcomes in endovascular aneurysm treatment.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Duswald, Tobias; Keith, Brendan; Lazarov, Boyan; Petrides, Socratis; Wohlmuth, Barbara
In: [PrePrint], arXiv, 2024.
@article{duswald24matern,
title = {Finite elements for Matérn-type random fields: Uncertainty in computational mechanics and design optimization},
author = {Tobias Duswald and Brendan Keith and Boyan Lazarov and Socratis Petrides and Barbara Wohlmuth},
editor = {arXiv},
url = {https://arxiv.org/abs/2403.03658},
doi = {https://doi.org/10.48550/arXiv.2403.03658},
year = {2024},
date = {2024-03-06},
urldate = {2024-03-06},
journal = {[PrePrint], arXiv},
abstract = {This work highlights an approach for incorporating realistic uncertainties into scientific computing workflows based on finite elements, focusing on applications in computational mechanics and design optimization. We leverage Matérn-type Gaussian random fields (GRFs) generated using the SPDE method to model aleatoric uncertainties, including environmental influences, variating material properties, and geometric ambiguities. Our focus lies on delivering practical GRF realizations that accurately capture imperfections and variations and understanding how they impact the predictions of computational models and the topology of optimized designs. We describe a numerical algorithm based on solving a generalized SPDE to sample GRFs on arbitrary meshed domains. The algorithm leverages established techniques and integrates seamlessly with the open-source finite element library MFEM and associated scientific computing workflows, like those found in industrial and national laboratory settings. Our solver scales efficiently for large-scale problems and supports various domain types, including surfaces and embedded manifolds. We showcase its versatility through biomechanics and topology optimization applications. The flexibility and efficiency of SPDE-based GRF generation empower us to run large-scale optimization problems on 2D and 3D domains, including finding optimized designs on embedded surfaces, and to generate topologies beyond the reach of conventional techniques. Moreover, these capabilities allow us to model geometric uncertainties of reconstructed submanifolds, such as the surfaces of cerebral aneurysms. In addition to offering benefits in these specific domains, the proposed techniques transcend specific applications and generalize to arbitrary forward and backward problems in uncertainty quantification involving finite elements.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Horvat, Medeea; Lunowa, Stephan B.; Sytnyk, Dmytro; Wohlmuth, Barbara
A lattice Boltzmann method for non-Newtonian blood flow in coiled intracranial aneurysms Artikel
In: [PrePrint], arXiv, 2024.
@article{horvat24lbm,
title = {A lattice Boltzmann method for non-Newtonian blood flow in coiled intracranial aneurysms},
author = {Medeea Horvat and Stephan B. Lunowa and Dmytro Sytnyk and Barbara Wohlmuth},
editor = {arXiv},
url = {https://arxiv.org/abs/2402.10809},
doi = {https://doi.org/10.48550/arXiv.2402.10809},
year = {2024},
date = {2024-02-16},
journal = {[PrePrint], arXiv},
abstract = {Intracranial aneurysms are the leading cause of stroke. One of the established treatment approaches is the embolization induced by coil insertion. However, the prediction of treatment and subsequent changed flow characteristics in the aneurysm, is still an open problem. In this work, we present an approach based on patient specific geometry and parameters including a coil representation as inhomogeneous porous medium. The model consists of the volume-averaged Navier-Stokes equations including the non-Newtonian blood rheology. We solve these equations using a problem-adapted lattice Boltzmann method and present a comparison between fully-resolved and volume-averaged simulations. The results indicate the validity of the model. Overall, this workflow allows for patient specific assessment of the flow due to potential treatment.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Holzberger, Fabian; Muhr, Markus; Wohlmuth, Barbara
In: [PrePrint], arXiv, 2024.
@article{holzberger24coiling,
title = {A Comprehensive Numerical Approach to Coil Placement in Cerebral Aneurysms: Mathematical Modeling and In Silico Occlusion Classification},
author = {Fabian Holzberger and Markus Muhr and Barbara Wohlmuth},
editor = {arXiv},
url = {https://arxiv.org/abs/2402.02798},
doi = {https://doi.org/10.48550/arXiv.2402.02798},
year = {2024},
date = {2024-02-05},
journal = {[PrePrint], arXiv},
abstract = {Endovascular coil embolization is one of the primary treatment techniques for cerebral aneurysms. Although it is a well established and minimally invasive method, it bears the risk of sub-optimal coil placement which can lead to incomplete occlusion of the aneurysm possibly causing recurrence. One of the key features of coils is that they have an imprinted natural shape supporting the fixation within the aneurysm. For the spatial discretization our mathematical coil model is based on the Discrete Elastic Rod model which results in a dimension-reduced 1D system of differential equations. We include bending and twisting responses to account for the coils natural curvature. Collisions between coil segments and the aneurysm-wall are handled by an efficient contact algorithm that relies on an octree based collision detection. The numerical solution of the model is obtained by a symplectic semi-implicit Euler time stepping method. Our model can be easily incorporated into blood flow simulations of embolized aneurysms. In order to differentiate optimal from sub-optimal placements, we employ a suitable in silico Raymond-Roy type occlusion classification and measure the local packing density in the aneurysm at its neck, wall-region and core. We investigate the impact of uncertainties in the coil parameters and embolization procedure. To this end, we vary the position and the angle of insertion of the microcatheter, and approximate the local packing density distributions by evaluating sample statistics.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Frank, Martin; Holzberger, Fabian; Horvat, Medeea; Kirschke, Jan; Mayr, Matthias; Muhr, Markus; Nebulishvili, Natalia; Popp, Alexander; Schwarting, Julian; Wohlmuth, Barbara
Numerical simulation of endovascular treatment options for cerebral aneurysms Artikel
In: [PrePrint], arXiv, 2024.
@article{frank24gamm,
title = {Numerical simulation of endovascular treatment options for cerebral aneurysms},
author = {Martin Frank and Fabian Holzberger and Medeea Horvat and Jan Kirschke and Matthias Mayr and Markus Muhr and Natalia Nebulishvili and Alexander Popp and Julian Schwarting and Barbara Wohlmuth},
editor = {arXiv},
url = {https://arxiv.org/abs/2402.00550},
doi = {https://doi.org/10.48550/arXiv.2402.00550},
year = {2024},
date = {2024-02-01},
journal = {[PrePrint], arXiv},
abstract = {Predicting the long-term success of endovascular interventions in the clinical management of cerebral aneurysms requires detailed insight into the patient-specific physiological conditions. In this work, we not only propose numerical representations of endovascular medical devices such as coils, flow diverters or Woven EndoBridge but also outline numerical models for the prediction of blood flow patterns in the aneurysm cavity right after a surgical intervention. Detailed knowledge about the post-surgical state then lays the basis to assess the chances of a stable occlusion of the aneurysm required for a long-term treatment success. To this end, we propose mathematical and mechanical models of endovascular medical devices made out of thin metal wires. These can then be used for fully resolved flow simulations of the post-surgical blood flow, which in this work will be performed by means of a Lattice Boltzmann method applied to the incompressible Navier-Stokes equations and patient-specific geometries. To probe the suitability of homogenized models, we also investigate poro-elastic models to represent such medical devices. In particular, we examine the validity of this modeling approach for flow diverter placement across the opening of the aneurysm cavity. For both approaches, physiologically meaningful boundary conditions are provided from reduced-order models of the vascular system. The present study demonstrates our capabilities to predict the post-surgical state and lays a solid foundation to tackle the prediction of thrombus formation and, thus, the aneurysm occlusion in a next step.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2023
Gjerde, Ingeborg G.; Kuchta, Miroslav; Rognes, Marie E.; Wohlmuth, Barbara
In: [PrePrint], arXiv, 2023.
@article{gjerde23perivascular,
title = {Directional flow in perivascular networks: Mixed finite elements for reduced-dimensional models on graphs},
author = {Ingeborg G. Gjerde and Miroslav Kuchta and Marie E. Rognes and Barbara Wohlmuth},
editor = {arXiv},
url = {https://arxiv.org/abs/2401.00484},
doi = {https://doi.org/10.48550/arXiv.2401.00484},
year = {2023},
date = {2023-12-31},
journal = {[PrePrint], arXiv},
abstract = {The flow of cerebrospinal fluid through the perivascular spaces of the brain is believed to play a crucial role in eliminating toxic waste proteins. While the driving forces of this flow have been enigmatic, experiments have shown that arterial wall motion is central. In this work, we present a network model for simulating pulsatile fluid flow in perivascular networks. We establish the well-posedness of this model in the primal and dual mixed variational settings, and show how it can be discretized using mixed finite elements. Further, we utilize this model to investigate fundamental questions concerning the physical mechanisms governing perivascular fluid flow. Notably, our findings reveal that arterial pulsations can induce directional flow in branching perivascular networks.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Holzberger, Fabian; Kirschke, Jan; Muhr, Markus; Nebulishvili, Natalia; Schwarting, Julian; Wohlmuth, Barbara
Breaking Blood Flow with Wires in Aneurysm Coiling Treatment Simulations Online
SIAM, News (Hrsg.): 2023, besucht am: 19.12.2023.
@online{holzberger23siam,
title = {Breaking Blood Flow with Wires in Aneurysm Coiling Treatment Simulations},
author = {Fabian Holzberger and Jan Kirschke and Markus Muhr and Natalia Nebulishvili and Julian Schwarting and Barbara Wohlmuth},
editor = {News SIAM},
url = {https://sinews.siam.org/Details-Page/breaking-blood-flow-with-wires-in-aneurysm-coiling-treatment-simulations},
year = {2023},
date = {2023-12-19},
urldate = {2023-12-19},
keywords = {},
pubstate = {published},
tppubtype = {online}
}
2022
Fritz, Marvin; Köppl, Tobias; Oden, John Tinsley; Wagner, Andreas; Wohlmuth, Barbara; Wu, Chengyue
A 1D–0D–3D coupled model for simulating blood flow and transport processes in breast tissue Artikel
In: International Journal for Numerical Methods in Biomedical Engineering, Bd. 38, Ausg. 7, S. e3612, 2022.
@article{fritz20221d,
title = {A 1D–0D–3D coupled model for simulating blood flow and transport processes in breast tissue},
author = {Marvin Fritz and Tobias Köppl and John Tinsley Oden and Andreas Wagner and Barbara Wohlmuth and Chengyue Wu},
editor = {Wiley Online Library},
url = {https://onlinelibrary.wiley.com/doi/abs/10.1002/cnm.3612},
doi = {https://doi.org/10.1002/cnm.3612},
year = {2022},
date = {2022-05-06},
urldate = {2022-05-06},
journal = {International Journal for Numerical Methods in Biomedical Engineering},
volume = {38},
issue = {7},
pages = {e3612},
abstract = {In this work, we present mixed dimensional models for simulating blood flow and transport processes in breast tissue and the vascular tree supplying it. These processes are considered, to start from the aortic inlet to the capillaries and tissue of the breast. Large variations in biophysical properties and flow conditions exist in this system necessitating the use of different flow models for different geometries and flow regimes. In total, we consider four different model types. First, a system of 1D nonlinear hyperbolic partial differential equations (PDEs) is considered to simulate blood flow in larger arteries with highly elastic vessel walls. Second, we assign 1D linearized hyperbolic PDEs to model the smaller arteries with stiffer vessel walls. The third model type consists of ODE systems (0D models). It is used to model the arterioles and peripheral circulation. Finally, homogenized 3D porous media models are considered to simulate flow and transport in capillaries and tissue within the breast volume. Sink terms are used to account for the influence of the venous and lymphatic systems. Combining the four model types, we obtain two different 1D–0D–3D coupled models for simulating blood flow and transport processes: The first model results in a fully coupled 1D–0D–3D model covering the complete path from the aorta to the breast combining a generic arterial network with a patient specific breast network and geometry. The second model is a reduced one based on the separation of the generic and patient specific parts. The information from a calibrated fully coupled model is used as inflow condition for the patient specific sub-model allowing a significant computational cost reduction. Several numerical experiments are conducted to calibrate the generic model parameters and to demonstrate realistic flow simulations compared to existing data on blood flow in the human breast and vascular system. Moreover, we use two different breast vasculature and tissue data sets to illustrate the robustness of our reduced sub-model approach.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
